Membrane biofouling in a wastewater nitrification reactor: Microbial succession from autotrophic colonization to heterotrophic domination
H. Lu, Z. Xue, P. Saikaly, S. P. Nunes, T. R. Bluver, W.-T. Liu
Water Research, 88, 337-345, (2016)
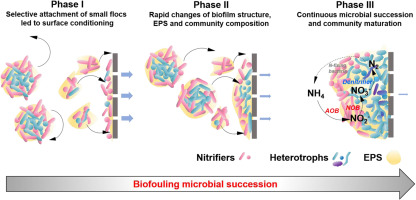
Membrane biofouling is a complex process that involves bacterial adhesion, extracellular polymeric substances (EPS) excretion and utilization, and species interactions. To obtain a better understanding of the microbial ecology of biofouling process, this study conducted rigorous, time-course analyses on the structure, EPS and microbial composition of the fouling layer developed on ultrafiltration membranes in a nitrification bioreactor. During a 14-day fouling event, three phases were determined according to the flux decline and microbial succession patterns. In Phase I (0–2 days), small sludge flocs in the bulk liquid were selectively attached on membrane surfaces, leading to the formation of similar EPS and microbial community composition as the early biofilms. Dominant populations in small flocs, e.g., Nitrosomonas, Nitrobacter, and Acinetobacter spp., were also the major initial colonizers on membranes. In Phase II (2–4 d), fouling layer structure, EPS composition, and bacterial community went through significant changes. Initial colonizers were replaced by fast-growing and metabolically versatile heterotrophs (e.g., unclassified Sphingobacteria). The declining EPS polysaccharide to protein (PS:PN) ratios could be correlated well with the increase in microbial community diversity. In Phase III (5–14 d), heterotrophs comprised over 90% of the community, whereas biofilm structure and EPS composition remained relatively stable. In all phases, AOB and NOB were constantly found within the top 40% of the fouling layer, with the maximum concentrations around 15% from the top. The overall microbial succession pattern from autotrophic colonization to heterotrophic domination implied that MBR biofouling could be alleviated by forming larger bacterial flocs in bioreactor suspension (reducing autotrophic colonization), and by designing more specific cleaning procedures targeting dominant heterotrophs during typical filtration cycles.